Newsletter
1 de July de 2025
New PPH Cycle Opens for Third Quarter of 2025 – Telecommunication Applications Excluded
In accordance with Ordinance 03/2025, published by the BPTO on March 25, 2025, a new limit of 800 Patent Prosecution Highway (PPH) requests is open for the third quarter of 2025.
Unlike the previous quarter, new PPH requests for applications with main IPC H04 (telecommunication) will not be accepted this quarter.
The BPTO will reassess the admittance of PPHs for telecommunication cases for the last quarter of 2025.
Up to this point in 2025, the BPTO has received 1118 PPH requests. The average time between the PPH request and the decision is 2,5 months.
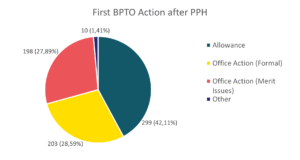
The graphic below shows the numbers and type of the first BPTO action after the PPH request. The general allowance rate for applications under PPH in 2025, including cases allowed after responding to Office Actions, is of 98,01%.
The major technical fields of the PPH requests were digital communication, medical technology, pharmaceuticals, and computing.
You may find more details on the new PPH rules in Brazil for 2025 in our previous newsletter by clicking on this link.
For further information, please do not hesitate to contact our team at mail@kasznarleonardos.com.
Last related news
2 de February de 2026
MP 1.335/2026: New Frameworks for Trademark Protection – FIFA Women’s World Cup 2027
The recently published Provisional Measure (MP) No. 1,335/2026—currently pending Congressional approval—introduces a distinct legal regime that significantly shifts the level of IP … MP 1.335/2026: New Frameworks for Trademark Protection – FIFA Women’s World Cup 2027

17 de December de 2025
Fast-track Examination Requests, including PPH, will be temporarily suspended in Brazil for Telecommunication Cases
By means of Ordinance 17/2025, published on December 16, 2025, the BPTO has announced that fast-track examination routes, including the Patent Prosecution … Fast-track Examination Requests, including PPH, will be temporarily suspended in Brazil for Telecommunication Cases

9 de December de 2025
Fake Software Resellers: A Growing Risk for Users and a New Front in IP Enforcement
For years, the most common form of software piracy involved downloading cracked or unauthorized versions from websites offering them for free. Although … Fake Software Resellers: A Growing Risk for Users and a New Front in IP Enforcement




