Newsletter
22 de December de 2023
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1.409 of 2023 approved the Regulatory Agenda (RA) which covers the priorities for the 2024-2025 biennium.
The Regulatory Agenda is a planning instrument for the normative activities of the Agency. It presents several priority topics to be regulated by ANVISA whilst its validity. Therefore, such Agenda sheds light on the themes do to be improved by regulatory means for the next two years in Brazil.
Social participation alongside the National Sanitary Surveillance System (Sistema Nacional de Vigilância Sanitária – SNVS) was crucial to the construction of the RA 24/25. There were 1.449 contributions received by ANVISA’s Public Consultancy.
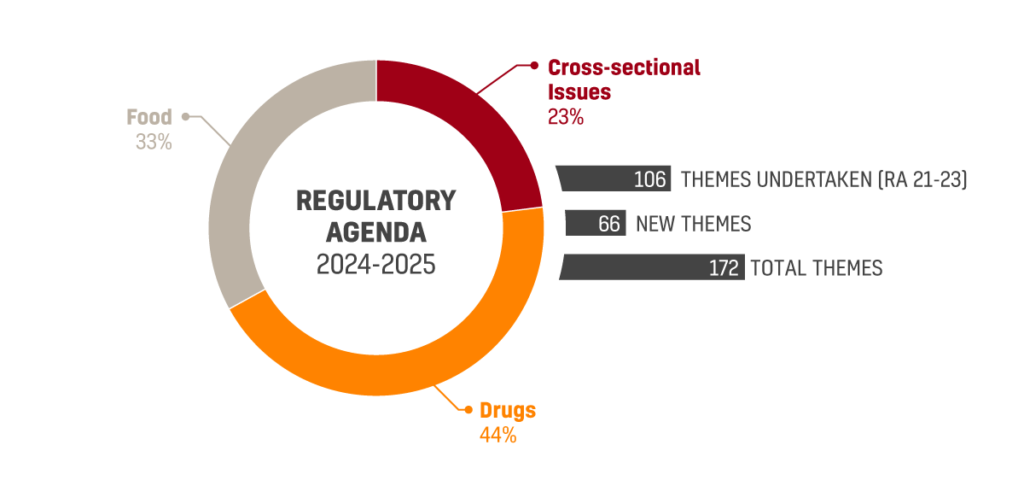
The just approved agenda covers 172 regulatory themes, divided into 16 macro themes. Topics that received most regulatory demands include Drugs with 45 themes, Food with 34 themes and cross-sectional issues with 23 themes.
106 out of the 172 themes pertaining the new agenda are related to the subject matters already announced on the 2021-2023 agenda. 66 themes are new and unrelated to the previous agenda.
The Regulatory Agenda 2024-2025 guarantees visibility and transparency for the normative regulatory activities by ANVISA. It will come into effect on January 2nd, 2024.
For more information regarding ANVISA, please do not hesitate to contact us by email: regulatorio@kasznarleonardos.com.
Last related news
2 de February de 2026
MP 1.335/2026: New Frameworks for Trademark Protection – FIFA Women’s World Cup 2027
The recently published Provisional Measure (MP) No. 1,335/2026—currently pending Congressional approval—introduces a distinct legal regime that significantly shifts the level of IP … MP 1.335/2026: New Frameworks for Trademark Protection – FIFA Women’s World Cup 2027

17 de December de 2025
Fast-track Examination Requests, including PPH, will be temporarily suspended in Brazil for Telecommunication Cases
By means of Ordinance 17/2025, published on December 16, 2025, the BPTO has announced that fast-track examination routes, including the Patent Prosecution … Fast-track Examination Requests, including PPH, will be temporarily suspended in Brazil for Telecommunication Cases

9 de December de 2025
Fake Software Resellers: A Growing Risk for Users and a New Front in IP Enforcement
For years, the most common form of software piracy involved downloading cracked or unauthorized versions from websites offering them for free. Although … Fake Software Resellers: A Growing Risk for Users and a New Front in IP Enforcement




