Newsletter
22 de December de 2023
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1.409 of 2023 approved the Regulatory Agenda (RA) which covers the priorities for the 2024-2025 biennium.
The Regulatory Agenda is a planning instrument for the normative activities of the Agency. It presents several priority topics to be regulated by ANVISA whilst its validity. Therefore, such Agenda sheds light on the themes do to be improved by regulatory means for the next two years in Brazil.
Social participation alongside the National Sanitary Surveillance System (Sistema Nacional de Vigilância Sanitária – SNVS) was crucial to the construction of the RA 24/25. There were 1.449 contributions received by ANVISA’s Public Consultancy.
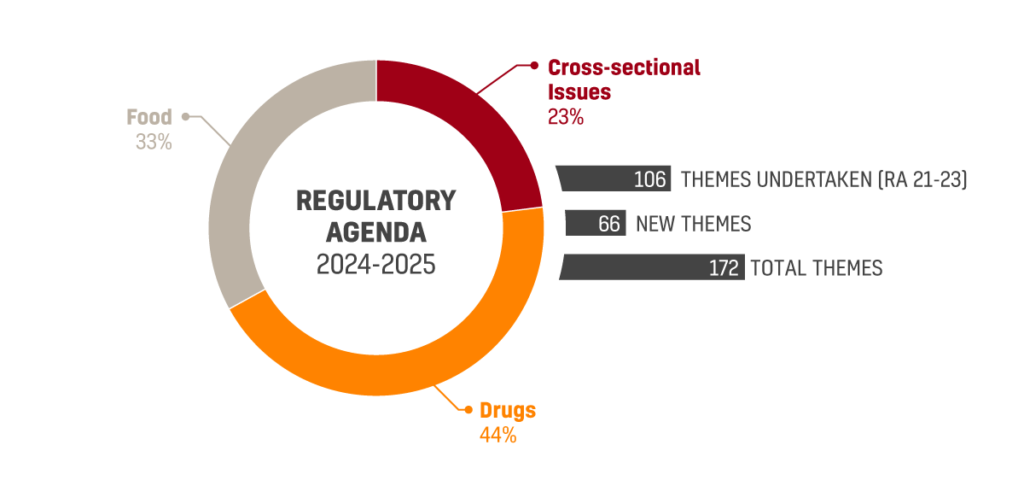
The just approved agenda covers 172 regulatory themes, divided into 16 macro themes. Topics that received most regulatory demands include Drugs with 45 themes, Food with 34 themes and cross-sectional issues with 23 themes.
106 out of the 172 themes pertaining the new agenda are related to the subject matters already announced on the 2021-2023 agenda. 66 themes are new and unrelated to the previous agenda.
The Regulatory Agenda 2024-2025 guarantees visibility and transparency for the normative regulatory activities by ANVISA. It will come into effect on January 2nd, 2024.
For more information regarding ANVISA, please do not hesitate to contact us by email: regulatorio@kasznarleonardos.com.
Last related news
1 de July de 2025
New PPH Cycle Opens for Third Quarter of 2025 – Telecommunication Applications Excluded
In accordance with Ordinance 03/2025, published by the BPTO on March 25, 2025, a new limit of 800 Patent Prosecution Highway (PPH) … New PPH Cycle Opens for Third Quarter of 2025 – Telecommunication Applications Excluded

1 de July de 2025
Brazil’s Supreme Court Partially Strikes Down Internet Law Provision, Imposes Stricter Duty of Care on Online Platforms
On Thursday, June 26, the Brazilian Supreme Federal Court (STF) issued a landmark ruling, partially invalidating Article 19 of the Brazilian Internet … Brazil’s Supreme Court Partially Strikes Down Internet Law Provision, Imposes Stricter Duty of Care on Online Platforms

24 de June de 2025
Software License Compliance: A Constitutional and Strategic Right
In Brazil, the holder of intellectual property rights is entitled not only to economically exploit their work, but also to monitor how … Software License Compliance: A Constitutional and Strategic Right




